MQSA and PerfectLum
- Home
- MQSA and PerfectLum
Ensure your mammography displays meet federal standards with our guide on MQSA and PerfectLum. Here, we break down what MQSA means, why it matters for your displays, and how to use PerfectLum to comply with these standards.
What Is MQSA?
The Mammography Quality Standards Act (MQSA) is a federal law enacted in 1992. Its purpose is to guarantee that every woman in the United States has access to high-quality mammography services for early breast cancer detection. To achieve this, MQSA:
- Sets Standards: Establishes national criteria for accreditation, certification, and inspection of mammography facilities.
- Ensures Quality: Mandates that facilities use equipment meeting strict quality benchmarks.
- Regulates Personnel: Requires that technologists and interpreting physicians have the proper training and qualifications.
- Mandates Inspections: Demands regular inspections to ensure compliance.
Key requirements for facilities include:
- Accreditation: Facilities must be accredited by an FDA-approved body.
- Certification: Facilities need certification from the FDA or a state agency acting on its behalf.
- Regular Inspections: Annual inspections confirm ongoing adherence to MQSA standards.
- Qualified Personnel and Up-to-Date Equipment: All staff and equipment must meet the defined quality standards.
For accreditation, facilities often work with trusted organizations like the American College of Radiology (ACR).
ACR Contact Information: The American College of Radiology 1891 Preston White Dr. Reston, VA 20191 1-800-227-6440
What Does MQSA Mean for Your Displays?
Under MQSA guidelines, mammography displays are vital components in the accurate evaluation of mammograms. These standards mean that:
- Quality Assurance (QA): Displays require regular QA testing to maintain consistent and reliable image quality.
- Manufacturer Alignment: Testing protocols should follow either the mammography unit’s recommended standards or approved alternative guidelines.
Comprehensive Documentation: Detailed records of test data and any corrective measures are necessary for annual MQSA inspections.
MQSA Using PerfectLum
PerfectLum is designed to simplify your display QA process and streamline compliance. Here’s how:
1. Performing the QA Test
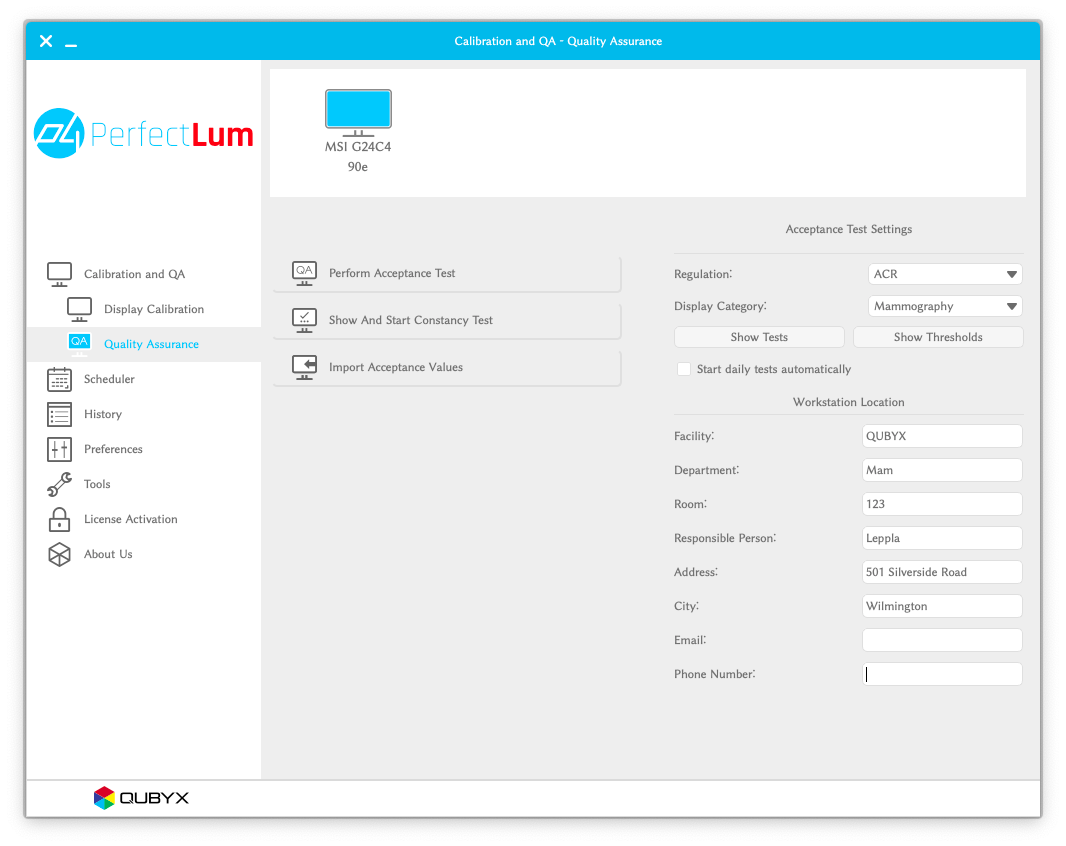
Use the integrated ACR Quality Assurance test available in PerfectLum:
- Setup:
- Connect a colorimeter (such as the PerfectLum S1) to your display.
- Launch PerfectLum:
- Open the PerfectLum application.
- Access QA Section:
- Navigate to the Tpa Quality Assurance section.
- Select Testing Parameters:
- Choose ACR as the testing method and Mammography as the category.
- Execute the Test:
- Click “Perform Acceptance Test” and follow the guided wizard.
2. Data Synchronization and Reporting
PerfectLum is fully integrated with the PerfectLum QA Server. When connected, all QA test data are automatically synchronized to the server, ensuring a centralized, secure repository for all your test results. This seamless integration makes it easy to:
- Access Consolidated Data: Keep all your test data in one place.
- Export Reports: Effortlessly export complete reports from the QA Server, simplifying the process of preparing documentation for your annual MQSA inspection.
3. Preparing for the Annual MQSA Inspection
In addition to performing regular QA tests and synchronizing data, ensure you compile the following information:
- QC Test Data:
- Complete test data as specified by your mammography unit manufacturer’s QC manual or the ACR DM QC Manual when using the alternative standard.
- Off-Site Equipment Records:
- QC test data for any display devices or equipment located off site.
- Documentation of Corrective Actions:
- Detailed records of any corrective measures implemented following QA tests.
4. Why Choose the ACR Test?
Using the ACR Digital Mammography Quality Control Manual for Full-Field Digital Mammography Systems (and its supplement for Digital Breast Tomosynthesis Mammography Systems) offers a proven alternative. Approved by the FDA as Alternative Standard #2423, this method provides:
- Flexible Standards: Facilities may choose between the manufacturer’s recommendations or the ACR alternative standard.
- Permanent Validity: The ACR alternative standard has no time limit, offering a consistent approach to quality assurance.
Using PerfectLum’s integrated ACR testing and QA server synchronization, you can ensure that your displays continuously meet ACR requirements and streamline your compliance documentation.
