News
- Home
- Display Calibration Compliance – Standards, Risks & Requirements

Display Calibration Compliance – Standards, Risks & Requirements
- December 13, 2025
- Sam Lee
Why Display Calibration Compliance Is A Requirement — Not a Preference
Executive Summary
Display calibration compliance is often misunderstood as a “quality improvement” or “best-practice” activity. In regulated imaging environments—particularly medical, dental, and diagnostic imaging—this assumption is incorrect.
Display calibration compliance is a requirement.
It is mandated, directly or indirectly, by international standards, clinical safety frameworks, and regulatory expectations. Failure to maintain calibrated displays introduces measurable clinical risk, regulatory exposure, and legal liability.
This technical insight explains why calibration is not optional, how compliance frameworks depend on it, and what organizations must implement to remain audit-ready.
1. What Display Calibration Compliance Actually Means (Technically)
Display Calibration Compliance is the controlled adjustment and verification of a display’s visual output so that it conforms to a defined standard.
At a technical level, Display calibration Compliance ensures:
-
Luminance accuracy (cd/m²)
-
Grayscale conformance (DICOM GSDF)
-
Color accuracy (ICC / perceptual rendering)
-
Gamma consistency
-
Temporal stability over time
Calibration is not a one-time setup. Displays drift due to panel aging, backlight decay, ambient conditions, and firmware changes.
Uncalibrated drift is non-deterministic, meaning clinicians cannot visually detect when accuracy is lost.
2. The Regulatory Foundations of Display Calibration Compliance
2.1 DICOM Part 14 – Grayscale Standard Display Function (GSDF)
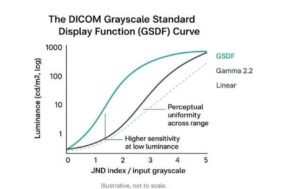
DICOM Part 14 defines how grayscale values must be rendered so that equal perceptual contrast is preserved across luminance levels.
This is critical for detecting:
-
Low-contrast lesions
-
Subtle density changes
-
Early-stage pathologies
Without Display calibration Compliance, GSDF conformance cannot be guaranteed.
2.2 Clinical Quality & Safety Standards
Display calibration compliance is embedded (explicitly or implicitly) in:
-
AAPM TG-18 / TG-270 (Medical imaging QC)
-
DIN 6868-157 (Diagnostic displays)
-
IEC 62563 (Medical display performance)
-
ISO 13485 (Medical device quality systems)
-
FDA & CE expectations for image fidelity
Auditors do not ask whether displays are calibrated.
They ask how, how often, and how it is documented.
3. Why “Visual Inspection” Is Not Display Calibration Compliance
A common operational misconception is that trained users can “see” display issues.
From a technical standpoint, this is false.
Human vision:
-
Adapts to ambient light
-
Masks gradual luminance decay
-
Cannot perceive gamma distortion reliably
-
Is influenced by prior exposure
Regulatory standards require instrument-based verification, not subjective assessment.
Calibration without measurement is not calibration.
4. Legal and Clinical Risk of Non-Calibrated Displays
4.1 Clinical Impact
Uncalibrated displays may:
-
Conceal low-contrast abnormalities
-
Alter grayscale perception
-
Distort color-dependent diagnostics
-
Reduce diagnostic confidence
These risks are systemic, not anecdotal.
4.2 Legal & Display Calibration Compliance Exposure
In post-incident reviews, display calibration compliance logs are often examined alongside:
-
PACS records
-
Image acquisition parameters
-
Radiologist reports
If displays were not demonstrably display calibration compliance:
-
Liability increases
-
Defense weakens
-
Accreditation may be challenged
Calibration documentation is legal evidence, not IT paperwork.
5. Calibration vs. Quality Control (QC): A Critical Distinction
| Activity | Purpose | Compliance Role |
|---|---|---|
| Calibration | Adjust display to standard | Mandatory |
| Quality Control | Verify ongoing performance | Mandatory |
| Profiling | Characterize device behavior | Supportive |
| Visual checks | User confidence | Insufficient |
Calibration establishes compliance.
QC maintains it.
Both are required.
6. Why OEM Settings Are Not Enough
Factory presets:
-
Do not account for site lighting
-
Do not reflect aging panels
-
Are not traceable to local policies
-
Cannot provide audit logs
Regulatory bodies require site-specific calibration, performed:
-
At acceptance
-
Periodically
-
After repairs
-
After environmental changes
7. The Role of Software-Driven Calibration Systems
Modern display calibration compliance programs rely on:
-
Automated calibration engines
-
Centralized compliance dashboards
-
Audit-ready reporting
-
Multi-OS compatibility
-
Hardware-agnostic support
Software-driven systems reduce:
-
Human error
-
Operational cost
-
Compliance gaps
-
Downtime
They also scale across enterprise imaging environments.
8. Display Calibration Compliance as an Organizational Control, Not an IT Task
Leading healthcare institutions treat display calibration complience as:
-
A clinical governance control
-
A risk-management mechanism
-
A regulatory safeguard
Ownership typically spans:
-
Clinical engineering
-
Imaging informatics
-
Quality assurance
-
Display Calibration Compliance management
When calibration is delegated informally, compliance fails systematically.
9. Key Takeaway
Display calibration complianc is not about making images “look better.”
It is about:
-
Clinical accuracy
-
Patient safety
-
Regulatory defensibility
-
Institutional credibility
In regulated imaging environments, calibration is not optional—it is foundational.
10. How QUBYX Software Enables Standards Display Calibration Complianc at Scale
QUBYX software solutions—most notably PerfectLum—are designed specifically to operationalize regulatory display calibration requirements across complex, multi-vendor environments. PerfectLum provides standards-based luminance and grayscale calibration aligned with DICOM Part 14 GSDF, IEC 62563, and AAPM guidance, enabling organizations to move from ad-hoc calibration practices to repeatable, auditable compliance workflows. By combining measurement-driven calibration, automated conformance checks, and centralized reporting, PerfectLum ensures that compliance is not dependent on individual users or local workstation conditions.
A critical advantage of the QUBYX approach is platform and vendor neutrality. PerfectLum and QUBYX OS Tools operate across Windows, macOS, and Linux, supporting diagnostic displays from multiple manufacturers without proprietary lock-in. This is particularly important for healthcare providers, OEMs, and system integrators who must manage heterogeneous fleets of displays while maintaining consistent compliance policies. Centralized compliance reporting allows calibration records, drift history, and verification results to be retained as audit-ready documentation, directly addressing regulatory and legal expectations.
In addition to commercial solutions, QUBYX actively contributes to and supports open-source calibration and color management frameworks, reinforcing transparency and long-term sustainability. These open standards and tools enable research institutions, OEM development teams, and regulated industries to validate workflows, integrate calibration into custom systems, and align internal quality controls with internationally recognized standards. Together, QUBYX commercial and open-source offerings form a compliance ecosystem—one that prioritizes accuracy, traceability, and regulatory defensibility over vendor-specific shortcuts.
CTA
Talk to our calibration experts
If you are responsible for diagnostic quality, regulatory readiness, or OEM imaging performance, our specialists can help you:
-
Design compliant calibration workflows
-
Implement enterprise-scale solutions
-
Prepare for audits and accreditation
-
Reduce clinical and legal risk
Start the conversation with our calibration experts today.
In a world where every Pixel accuracy matters, PerfectLum by QUBYX proves that innovation can deliver clinical precision without financial compromise. It’s not just calibration—it’s the democratization of diagnostic imaging.
To secure Medical Display Quality Assurance with precision while reducing the recurring costs of proprietary hardware, the answer is clear: transition to a Calibration Software platform with QUBYX OS Tools (Free) and PerfectLum today. Now, you easily pay less for Radiology.
Tags:
display calibration compliance, DICOM GSDF, medical display calibration, color calibration standards, diagnostic display quality control
Related Posts
- February 24, 2026
- News
What is AAPM TG270? A Practical Guide to AAPM TG-270
- February 24, 2026
- News
How to Calibrate a Display to DICOM GSDF with PerfectLum
- February 23, 2026
- News
Why Calibrate a Display to DICOM? And the Role




