News
- Home
- Integrated Quality Assurance Compliance Monitoring | Qubyx

Integrated Quality Assurance Compliance Monitoring | Qubyx
- February 3, 2026
- Shamsul
Integrated Quality Assurance Compliance Monitoring for Diagnostic Imaging
Introduction
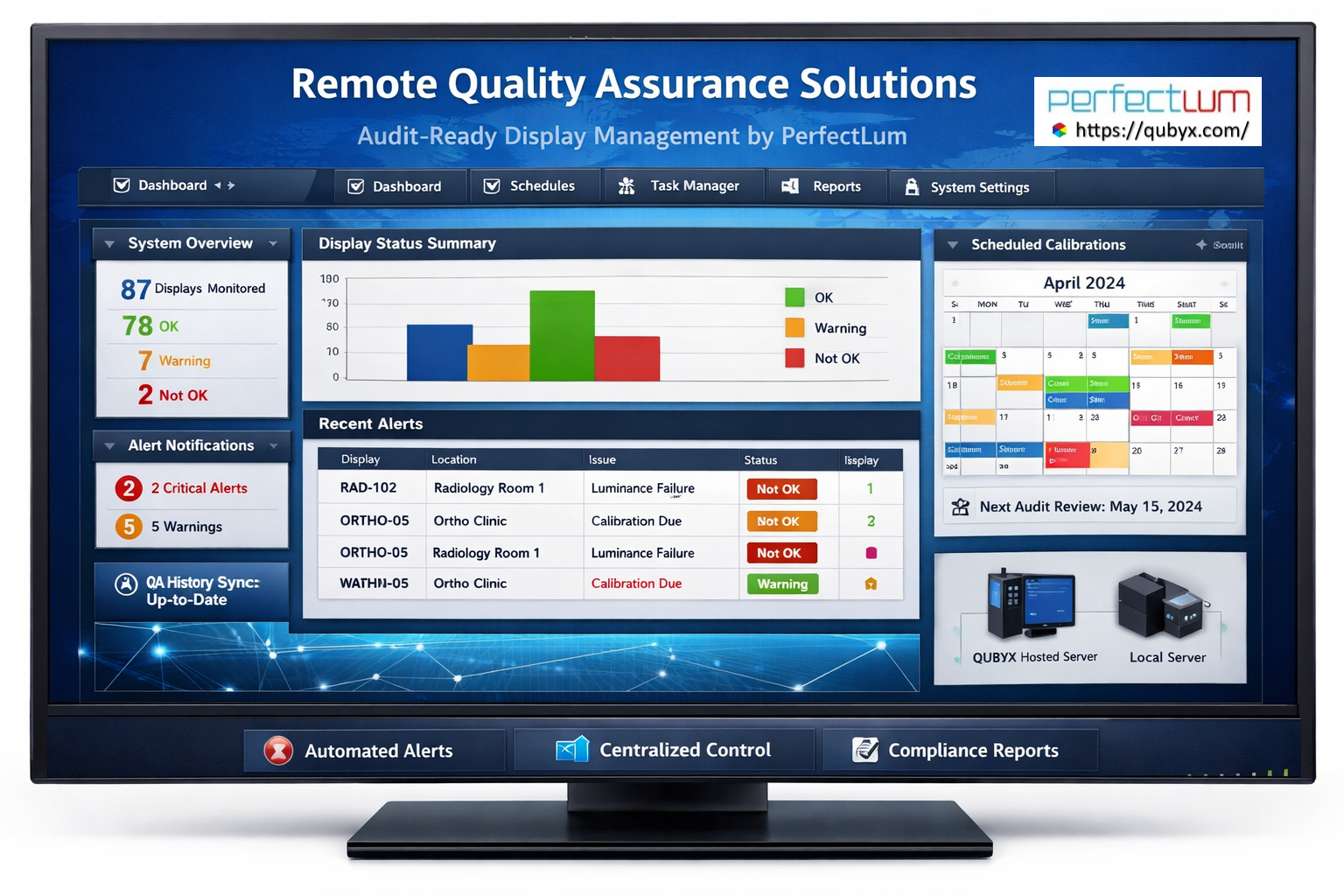
In regulated medical imaging environments, quality is not a one-time activity—it is a continuous obligation. Integrated quality assurance compliance monitoring has become essential for organizations that must demonstrate consistent performance, regulatory adherence, and audit readiness across complex imaging infrastructures.
At Qubyx, integrated quality assurance compliance monitoring is engineered into the core of the platform. By combining rigorous acceptance testing with automated, scheduled consistency checks, Qubyx enables imaging organizations to meet—and exceed—the most demanding global standards with confidence and efficiency.
What Is Integrated Quality Assurance Compliance Monitoring?
Integrated quality assurance compliance monitoring refers to a unified approach where quality assurance (QA) processes and regulatory compliance checks operate as a single, automated system rather than isolated, manual tasks.
Instead of relying on periodic testing or fragmented tools, integrated quality assurance compliance monitoring ensures that:
-
Acceptance testing is fully documented and validated
-
Ongoing consistency checks are automatically scheduled
-
Performance data is centrally stored and auditable
-
Compliance with regional and international standards is continuously maintained
This integrated model reduces operational risk, eliminates compliance gaps, and provides defensible proof of quality over the full system lifecycle.
Engineered for Acceptance Testing and Ongoing Consistency
A foundational element of integrated quality assurance compliance monitoring is the seamless transition from system acceptance to daily operation.
Qubyx platforms are designed to support:
-
Comprehensive acceptance testing at installation
-
Automatically scheduled consistency checks throughout system use
-
Continuous performance validation without manual intervention
By embedding these processes into a single workflow, integrated quality assurance compliance monitoring ensures that quality is not dependent on human memory, spreadsheets, or ad-hoc testing routines.
Standards-Driven by Design
Effective integrated quality assurance compliance monitoring must be aligned with recognized clinical and regulatory frameworks. Qubyx supports a wide range of globally respected standards to ensure diagnostic integrity, patient safety, and regulatory readiness.
Supported Standards and Protocols
-
DICOM calibration validated and certified
Ensures accurate grayscale and luminance response for diagnostic displays. -
AAPM TG18 & AAPM TG270
Tailored for precise dosimetry, imaging performance evaluation, and radiology quality control. -
NYC PDM & NY PDM (New York State)
Designed to meet stringent local performance, safety, and inspection requirements. -
DIN 6868-157
Full compliance with German standards for medical display quality control. -
MQSA Monitor Inspection
Supports adherence to the Mammography Quality Standards Act for reliable breast imaging diagnostics. -
ACR (American College of Radiology)
Upholds the rigorous QA and performance expectations required for accredited imaging facilities.
Through integrated quality assurance compliance monitoring, these standards are not treated as isolated checklists—but as continuously enforced requirements.
Automation That Eliminates Compliance Risk
Manual QA processes introduce variability, delay, and audit vulnerability. Integrated quality assurance compliance monitoring replaces manual workflows with automation that is predictable, traceable, and repeatable.
Key automation capabilities include:
-
Automatic test scheduling
QA tests are executed on time, every time, without staff intervention. -
Remote QA server architecture
Centralized monitoring across single or multi-site imaging environments. -
Complete reporting and historical database
Every test result is stored, searchable, and audit-ready. -
Fast calibration workflows
Minimizes system downtime while maintaining compliance. -
Easy installation and usability
Reduces training overhead while increasing adoption consistency.
Automation ensures that integrated quality assurance compliance monitoring functions as a safeguard—not an administrative burden.
Centralized Reporting and Audit Readiness
Regulators and accreditation bodies increasingly expect objective, longitudinal evidence of compliance. Integrated quality assurance compliance monitoring provides this evidence by design.
With centralized reporting and historical data retention, organizations can:
-
Demonstrate compliance during audits without last-minute data collection
-
Track performance trends over time
-
Identify early signs of drift or degradation
-
Support internal quality governance programs
This level of transparency transforms QA from a reactive task into a strategic operational asset.
Built for Distributed and Modern Imaging Environments
Modern diagnostic imaging is no longer confined to a single reading room. Integrated quality assurance compliance monitoring must support:
-
Hospitals and enterprise imaging networks
-
Teleradiology and distributed reading environments
-
Multi-vendor and multi-modality infrastructures
Qubyx platforms are architected to scale across these environments while maintaining consistent enforcement of standards, policies, and documentation.
Why Integrated Quality Assurance Compliance Monitoring Matters
Without integrated quality assurance compliance monitoring, organizations face:
-
Increased audit risk
-
Inconsistent display performance
-
Manual workload and human error
-
Difficulty proving regulatory compliance
By contrast, an integrated approach delivers:
-
Continuous validation against industry standards
-
Reduced operational burden
-
Defensible, repeatable compliance
-
Long-term reliability and trust in diagnostic quality
Conclusion
In today’s regulated imaging landscape, quality assurance and compliance cannot operate in silos. Integrated quality assurance compliance monitoring provides a unified, automated framework that ensures acceptance testing, consistency checks, reporting, and standards compliance function together as a single system.
With Qubyx, integrated quality assurance compliance monitoring is not an add-on—it is the foundation. From installation to everyday operation, every test is executed automatically, every result is documented, and every system remains aligned with the highest global benchmarks for diagnostic imaging excellence.
Start the conversation with our calibration experts today.
In a world where every Pixel accuracy matters, PerfectLum by QUBYX proves that innovation can deliver clinical precision without financial compromise. It’s not just calibration—it’s the democratization of diagnostic imaging.
About the Author:
Shamsul Islam is a strategy and growth professional focused on regulated B2B technology markets. He supports QUBYX LLC and its medical imaging solutions through product positioning, go-to-market strategy, and end-to-end digital content development, including website, social media, and educational video initiatives aligned with quality, compliance, and governance-driven environments.
Tags:
integrated quality assurance compliance monitoring, automated imaging QA, diagnostic imaging compliance, medical display QA, regulatory imaging standards
Related Posts
- February 4, 2026
- News
QUBYX Software Reseller Program Start Your Reseller Journey Today
- February 3, 2026
- News
Integrated Quality Assurance Compliance Monitoring for Diagnostic Imaging Introduction