News
- Home
- QUBYX Viewer: Quality Gate for Medical & Industrial Imaging

QUBYX Viewer: Quality Gate for Medical & Industrial Imaging
- December 17, 2025
- Shamsul
QUBYX Viewer as a Quality Gate in Medical & Industrial Imaging
In both medical diagnostics and industrial inspection, images are not “just visuals.” They are evidence. A single missed grayscale detail in a chest radiograph or a barely visible defect in a weld X-ray can change outcomes, trigger rework, delay production, or—most critically—introduce risk.
That is why leading organizations treat imaging not as a passive step, but as a controlled process with defined checkpoints. One of the most effective checkpoints is a quality gate: a standardized stage in the workflow where images are reviewed, validated, and either approved to proceed or flagged for corrective action.
QUBYX Viewer can serve precisely this role—a practical, enforceable quality gate that helps teams ensure images are displayed consistently, reviewed reliably, and assessed against documented quality criteria in both medical and industrial imaging environments.
What “Quality Gate” Means in Imaging Workflows
A quality gate is a control point designed to prevent downstream errors. In imaging, it typically verifies that:
-
The right study/asset is being reviewed (patient/case/job integrity)
-
The image quality is sufficient for the intended purpose
-
Display presentation is consistent (luminance, grayscale response, ambient conditions)
-
Review steps are performed with repeatable criteria
-
Findings are documented and escalated correctly
A quality gate is not only about catching defects. It is about ensuring confidence—that decisions are made on images that are reliably rendered and systematically assessed.
Why Quality Gates Matter More Than Ever
1) Growing imaging volumes and distributed teams
Radiology departments, telehealth networks, and industrial inspection operations increasingly rely on distributed reviewers. Without a consistent viewing and validation layer, variability creeps in—especially across different locations, displays, and lighting conditions.
2) Higher stakes and tighter tolerances
In healthcare, display inconsistency can affect subtle contrast perception. In manufacturing and NDT (non-destructive testing), tolerance for missed defects is extremely low, and auditability requirements keep increasing.
3) Compliance and customer expectations
Healthcare environments operate under strict expectations for display performance and traceable processes. Industrial organizations face customer audits, certification standards, and internal QMS requirements.
A quality gate approach—supported by the right viewer—creates a defensible, repeatable workflow.
How QUBYX Viewer Functions as a Quality Gate
1) Standardized image review for consistent decisions
A core requirement of a quality gate is standardization. QUBYX Viewer helps provide a consistent review experience across teams by reducing variability in how images are presented and assessed. This is vital when multiple stakeholders interpret the same imaging evidence—radiologists, technicians, QA teams, engineers, and external auditors.
Outcome: Fewer interpretation differences driven by viewing conditions rather than image content.
2) A “verification layer” between acquisition and decision-making
In many workflows, images move from acquisition directly into reporting, archiving, or acceptance—sometimes without a robust verification step. A dedicated review stage supported by QUBYX Viewer enables teams to confirm:
-
Image completeness (all necessary views captured)
-
Visibility of critical details
-
Appropriateness of the study for diagnostic/inspection purpose
-
Readiness for downstream reporting or acceptance
Outcome: Issues are caught early, before they become clinical risk, production delays, or customer complaints.
3) Display integrity as a prerequisite for image integrity
Even perfect images can be interpreted incorrectly if they are displayed poorly. A true quality gate considers not only the file, but the presentation.
In medical imaging, consistent grayscale presentation is a recognized requirement for dependable reading—commonly associated with DICOM GSDF behavior and controlled luminance performance. In industrial imaging, consistent contrast rendering and stable display characteristics can determine whether micro-defects are visible.
Outcome: The gate validates not just “the image,” but the conditions under which the image is judged.
4) Traceability and repeatability for QA and audits
Quality gates are valuable because they can be documented. In regulated or audited environments, you need to demonstrate that:
-
Review steps exist
-
Criteria are consistent
-
Exceptions are flagged and handled
-
Decisions are defensible
Using a viewer as a controlled checkpoint supports a more systematic QA posture, especially when integrated into a broader quality management system (QMS) or clinical governance process.
Outcome: A stronger audit trail and fewer “ad hoc” review practices.
Use Case 1: Medical Imaging Quality Gate
Where QUBYX Viewer fits
QUBYX Viewer can sit at key points in medical imaging workflows such as:
-
Pre-reporting review: confirm study quality before diagnostic interpretation
-
Peer review and second reads: reduce variability across readers
-
QA rounds: standardized display review for training and governance
-
Multi-site consistency: align review across different clinics and workstations
What it prevents
-
Loss of subtle grayscale detail due to inconsistent display behavior
-
Variability caused by uncontrolled ambient light and non-standard monitors
-
Risk introduced by inconsistent viewing configurations across sites
-
Re-reading and repeat imaging driven by avoidable quality issues
Why it matters
In high-impact modalities—radiology, mammography, CT/MR, nuclear medicine—the smallest contrast differences can influence decisions. A quality gate supported by a controlled viewer helps protect diagnostic confidence and patient safety.
Use Case 2: Industrial Imaging Quality Gate
Where QUBYX Viewer fits
Industrial imaging workflows benefit from a viewer-based quality gate in areas like:
-
NDT inspection validation (welds, castings, pipelines, aerospace components)
-
Incoming quality control for components requiring imaging proof
-
Process verification where images confirm production integrity
-
Customer acceptance packages where reviewed images form part of deliverables
What it prevents
-
Missed defects due to inconsistent contrast rendering
-
Disputes when customers and suppliers view “the same image” differently
-
Rework and downtime caused by late-stage defect discovery
-
Non-conformances due to weak documentation and review consistency
Why it matters
Industrial inspection depends on repeatable visual evidence. A quality gate ensures the evidence is consistently presented, reliably reviewed, and properly documented.
Practical Implementation: How to Deploy QUBYX Viewer as a Quality Gate
Step 1: Define pass/fail criteria
Document what “acceptable” means for your images. Examples:
-
Minimum visibility thresholds for key structures/defects
-
Required views or sequences per study/inspection
-
Common failure modes (under/over exposure, motion blur, incomplete coverage)
Step 2: Standardize review conditions
-
Control ambient lighting where possible
-
Align display settings and behavior across workstations
-
Ensure consistent grayscale/contrast behavior for the task
Step 3: Position the gate in the workflow
Typical placements:
-
Immediately after acquisition (catch issues early)
-
Before reporting/acceptance (protect decisions)
-
Before customer delivery (avoid disputes)
Step 4: Create escalation pathways
When the gate fails, what happens?
-
Repeat acquisition?
-
Secondary review?
-
Engineering escalation?
-
Corrective action record in QMS?
Step 5: Measure outcomes
Track:
-
Rework rates
-
Repeat imaging rates
-
Review turnaround times
-
Disputes/returns
-
Audit findings
Why QUBYX Viewer Is a Strategic Choice for Quality-Driven Organizations
A viewer used as a quality gate is not just software—it becomes part of your operational risk control. Organizations adopt this approach to achieve:
-
Consistency: Less variability between reviewers and locations
-
Reliability: More dependable decisions based on stable presentation
-
Efficiency: Fewer repeats, less rework, smoother throughput
-
Governance: Stronger QA structure and audit readiness
-
Scalability: Standardized review across departments, sites, and partners
Conclusion: A Quality Gate Protects Outcomes, Not Just Images
Whether your objective is diagnostic confidence in healthcare or defect detection in industrial inspection, the principle is the same: you cannot separate image interpretation from the conditions in which the image is displayed and reviewed.
By positioning QUBYX Viewer as a quality gate, organizations can standardize review, reduce variability, strengthen QA, and create a more defensible imaging workflow—where decisions are backed by consistent, controlled, and repeatable visual evidence.
CTA
If you want to standardize imaging review across sites, teams, or production lines, consider implementing QUBYX Viewer as a formal quality gate in your workflow. It is a practical step toward measurable improvements in consistency, compliance readiness, and decision confidence.
Start the conversation with our calibration experts today.
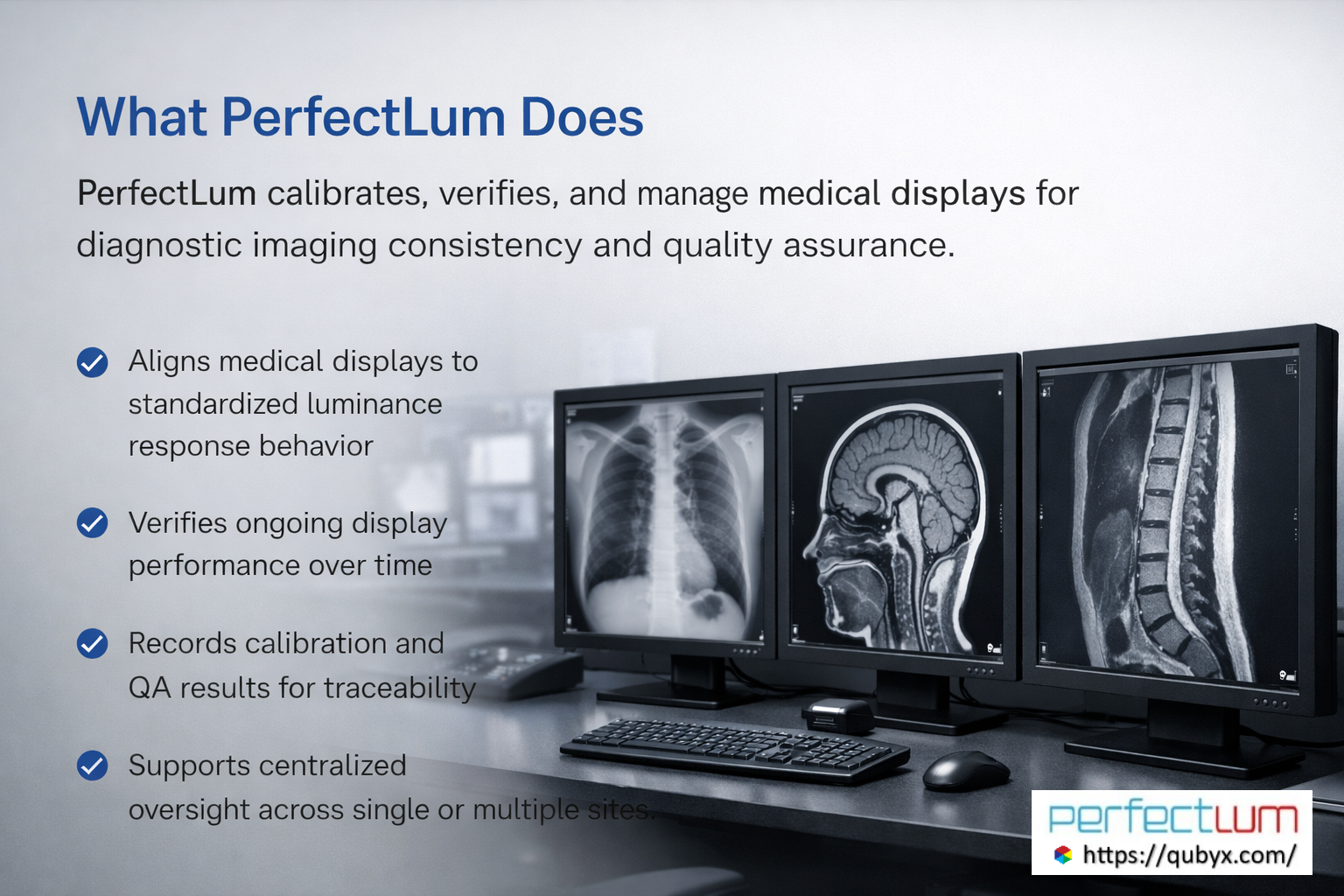
In a world where every Pixel accuracy matters, PerfectLum by QUBYX proves that innovation can deliver clinical precision without financial compromise. It’s not just calibration—it’s the democratization of diagnostic imaging.
To secure Medical Display Quality Assurance with precision while reducing the recurring costs of proprietary hardware, the answer is clear: transition to a Calibration Software platform with QUBYX OS Tools (Free) and PerfectLum today. Now, you easily pay less for Radiology.
Ready to dive in? Explore the code and documentation on GitHub:
Tags:
QUBYX Viewer, imaging quality gate, medical imaging QA, industrial imaging QA, DICOM viewer quality control, DICOM GSDF compliance, display calibration QA, diagnostic display review, visual inspection workflows, imaging quality assurance, radiology workflow validation, non-destructive testing imaging
Related Posts
- January 26, 2026
- News
Core Capabilities of Display Calibration and QA Management Display
- January 25, 2026
- News
Display Standardization in Global Teleradiology Global teleradiology has transformed
- January 24, 2026
- News
How PerfectLum Is Redefining Teleradiology Quality Assurance Why Quality