News
- Home
- PerfectLum FDA 510k clearance – Medical Imaging Beyond Traditional Boundaries
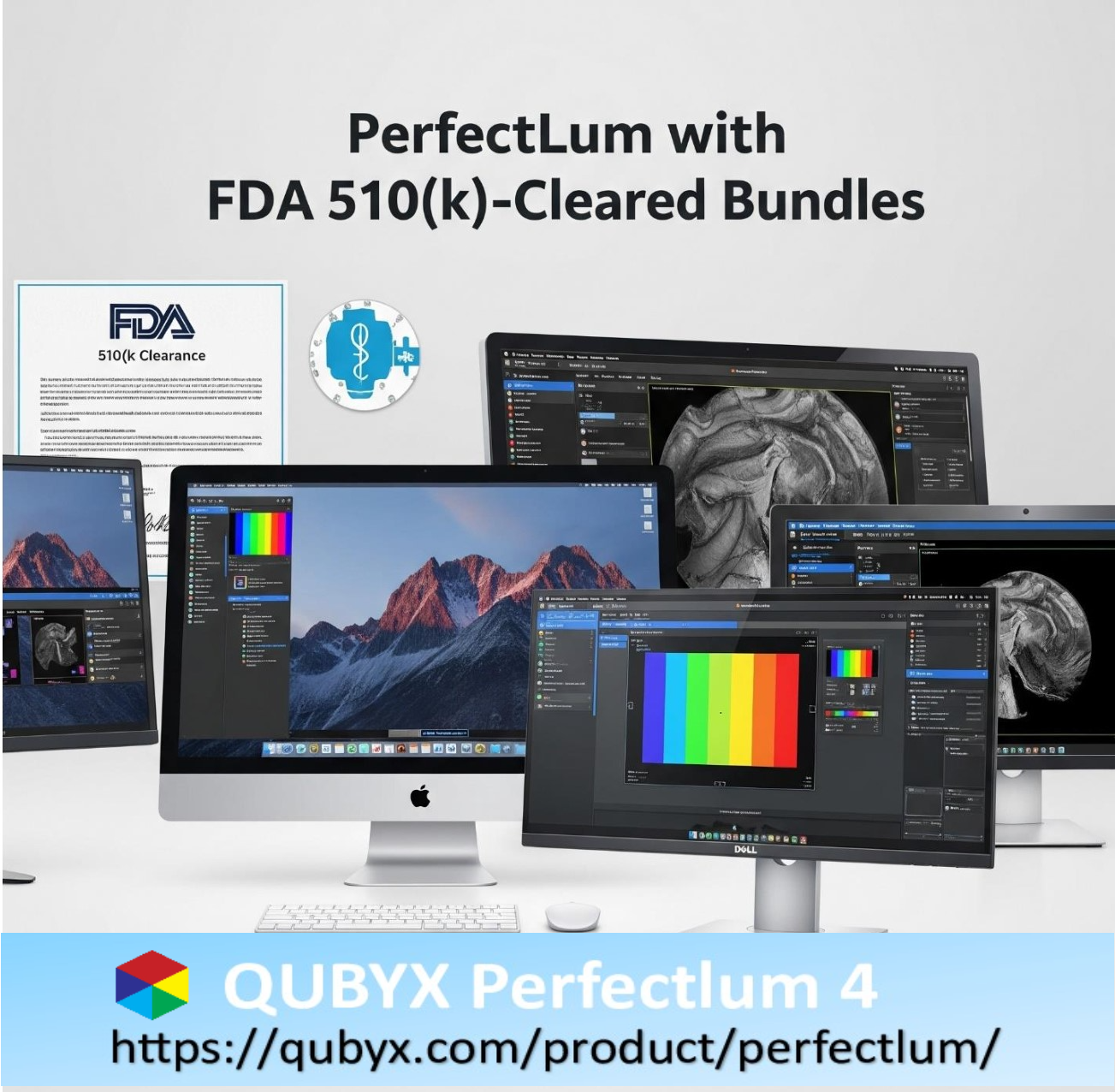
PerfectLum FDA 510k clearance – Medical Imaging Beyond Traditional Boundaries
- August 15, 2025
- Shamsul
PerfectLum FDA 510(k) Clearance
Expanding Medical Imaging Beyond Traditional Boundaries
In diagnostic imaging, accuracy and compliance are non-negotiable. The quality of the display you use directly impacts diagnostic confidence and patient safety. Traditionally, hospitals have relied exclusively on medical-grade monitors—but with advancements in software calibration and QA technology, the possibilities have expanded. PerfectLum FDA510(k) Clearance made is possible.
PerfectLum, developed by QUBYX Software Technologies, has achieved FDA 510(k) clearance for specific bundled configurations with both medical displays (like LG healthcare monitors) and consumer-grade displays (such as Dell UltraSharp and Apple iMac). This breakthrough gives healthcare professionals new flexibility while maintaining the rigorous standards required for clinical use.
What Does FDA 510(k) Clearance Mean?
The FDA 510(k) process is a regulatory pathway that verifies a medical device—or in this case, a monitor-software bundle—is substantially equivalent to an already approved device for the same use.
For display calibration and QA, this means:
- The hardware + PerfectLum software combination has been tested and validated for diagnostic radiology.
- Performance and image accuracy meet the standards necessary for medical image interpretation.
- Healthcare facilities can confidently deploy these approved bundles without sacrificing compliance or image quality.
PerfectLum’s Approved Bundles
1- Dell UltraSharp Displays + PerfectLum
QUBYX secured FDA 510(k) clearance for certain Dell UltraSharp models bundled with PerfectLum.
- Example: Dell UP3216Q + PerfectLum FDA 510(k) cleared for diagnostic use.
- Benefits: High-resolution panels, affordability, and PerfectLum’s precision calibration bring consumer-grade hardware into the clinical arena.
2- Apple iMac + PerfectLum
Apple’s 27-inch Retina 5K iMac with pairing with PerfectLum, has received FDA clearance for diagnostic imaging.
- Benefits: macOS compatibility, excellent color reproduction, and the ability to use a sleek all-in-one setup for teleradiology or remote QA.
- This is a game-changer for Mac-based healthcare facilities and radiologists who prefer Apple hardware.
3- LG Medical Displays + PerfectLum
LG’s professional-grade medical monitors—such as the LG 21HK512D or LG 32HL512D—are available with PerfectLum as part of PerfectLum FDA 510(k)-cleared bundles.
- Benefits: Built-for-healthcare design, factory DICOM calibration, and integration with PerfectLum for continuous QA and compliance reporting.
Why This Matters for Medical Imaging
Before FDA-cleared bundles like these, remote radiologists and smaller clinics faced two choices:
- Invest in expensive medical-grade monitors.
- Risk non-compliance and inaccurate images on consumer-grade displays.
PerfectLum FDA 510(k)-cleared configurations bridge that gap, offering:
- Regulatory compliance for approved bundles.
- Cost savings without compromising image quality.
- Cross-platform flexibility (Windows and macOS).
- Remote QA capabilities—perfect for teleradiology.
PerfectLum FDA 510(k) Features That Ensure Clinical Readiness
- DICOM GSDF Calibration – Guarantees grayscale display performance meets medical imaging standards.
- Automated QA Scheduling – Ensures constancy checks and calibration run on time.
- Web-Based QA Management – Manage, track, and verify calibration results across multiple sites.
- Audit-Ready Logs – Keep compliance documentation accessible for inspections.
- Cross-Platform Support – Works seamlessly on Windows PCs, medical workstations, and Apple iMac.
Important Note on Compliance
While PerfectLum supports many monitors, PerfectLum FDA 510(k) clearance applies to specific hardware + software bundles. Using PerfectLum on a non-cleared monitor can still improve image quality, but it may not be legally approved for diagnostic reading.
Always:
- Check your exact model and bundle in the FDA 510(k) database.
- Document your QA and calibration process.
- Follow your facility’s compliance policies.
Conclusion
PerfectLum FDA 510(k)–cleared bundles with LG medical displays, Dell UltraSharp monitors, and Apple iMac open up new opportunities for healthcare facilities to combine clinical-grade accuracy with cost-effective, flexible hardware choices.
Whether you’re upgrading a hospital reading room, setting up a remote teleradiology station, or standardizing QA across multiple sites, PerfectLum ensures your displays remain diagnostically reliable, compliant, and ready for audits—on both medical and consumer-grade hardware.
Ready to elevate your imaging accuracy? Visit qubyx.com to learn more about PerfectLum and how it can transform your iMac into a trusted partner in diagnostic imaging.
Keywords: PerfectLum FDA 510(k) clearance, Dell UltraSharp medical imaging, Apple iMac DICOM calibration, LG medical monitor QA, PerfectLum MacOS, medical display calibration software, teleradiology compliance, PerfectLum FDA 510(k),
Related Posts
- February 5, 2026
- News
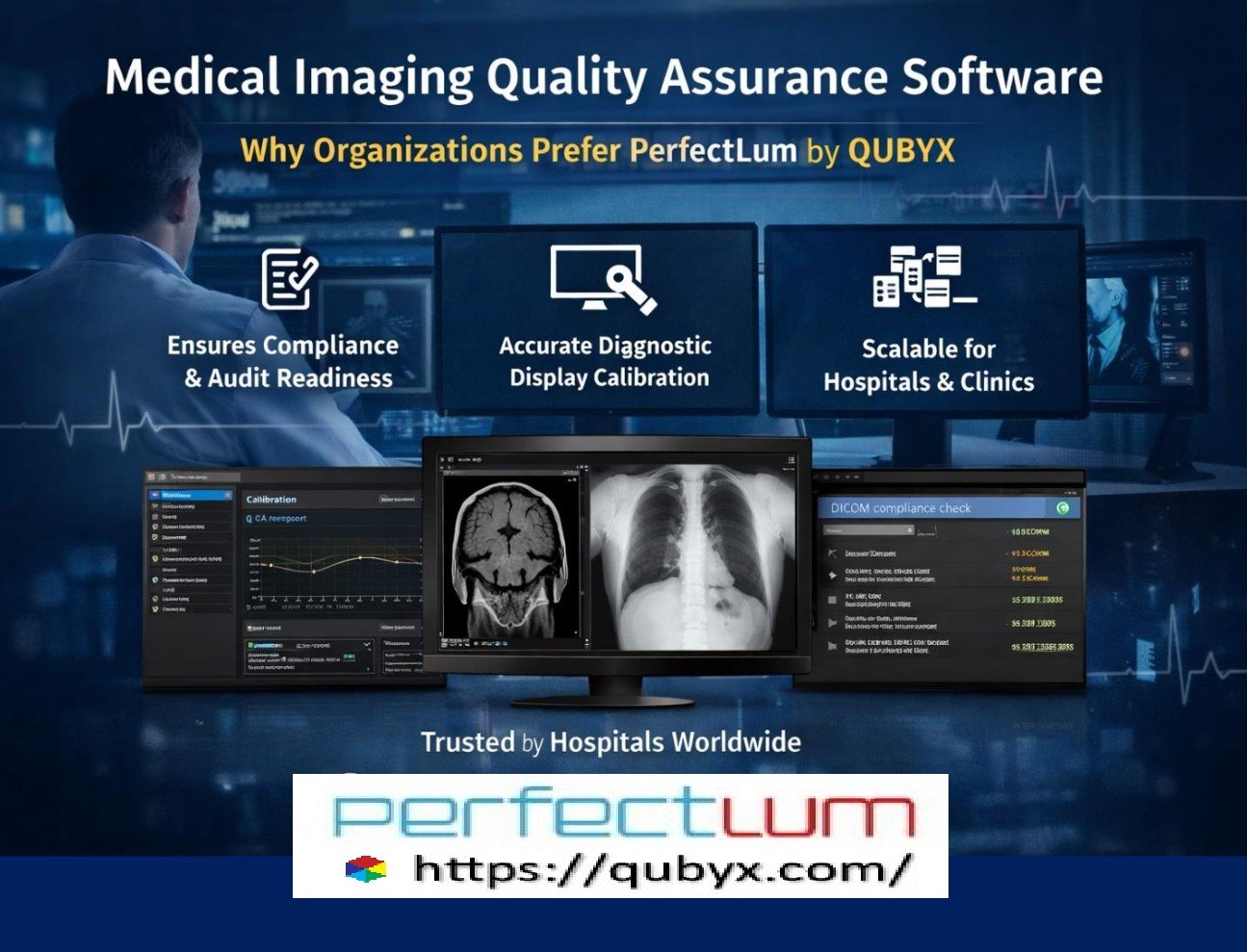
Medical Imaging Quality Assurance Software Why Organizations Prefer PerfectLum by
- February 4, 2026
- News
QUBYX Software Reseller Program Start Your Reseller Journey Today
- February 3, 2026
- News
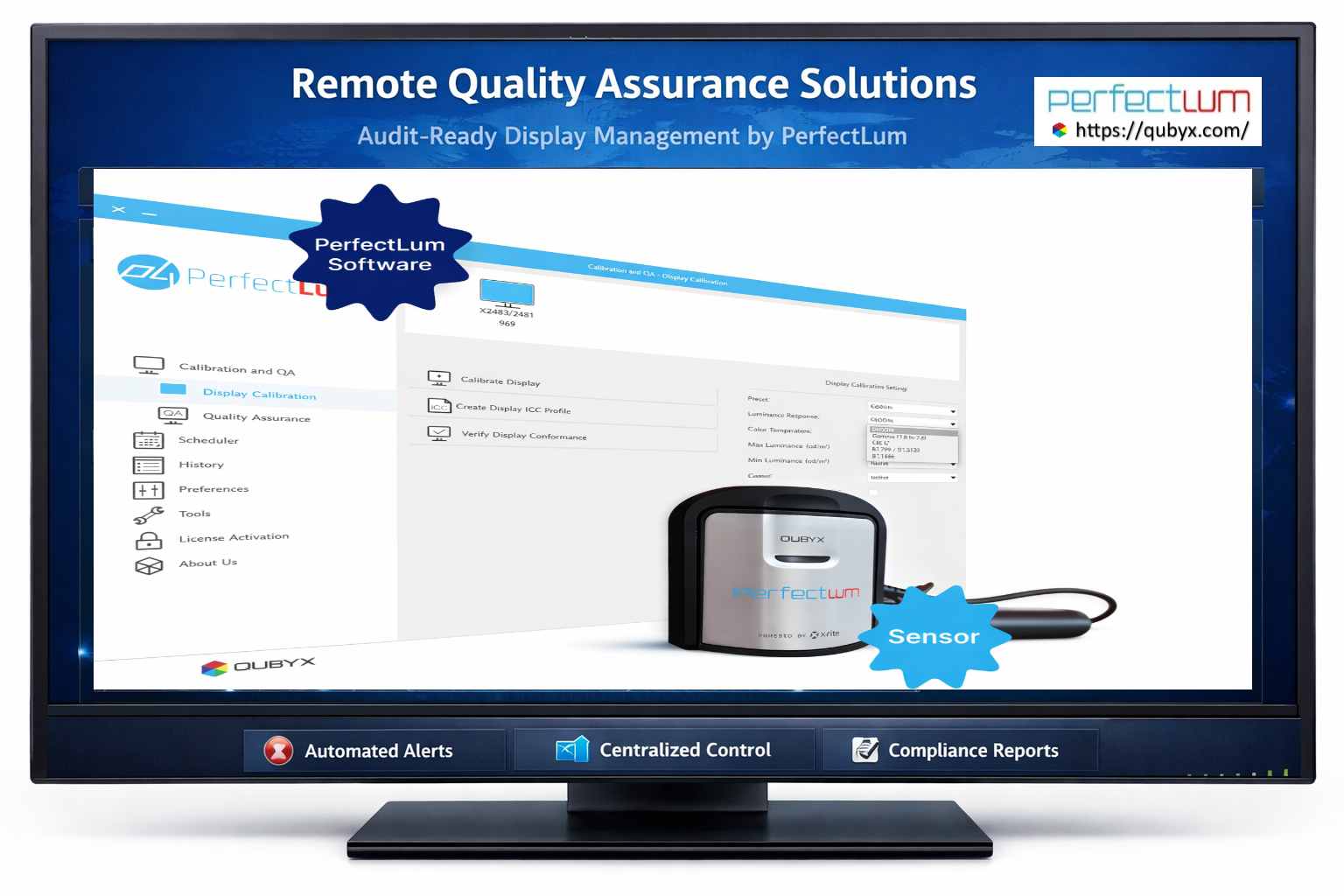
Elevate Your Standards with Remote Quality Assurance Solutions In